Description
Avacen 100 - Demo unit - slightly used with new cuff
Do you seek pain relief and muscle relaxation without side effects? Are you living with the pain of chronic arthritis joint pain? Are you dissatisfied with your current pain treatment regimen?
Advanced Vascular Circulation Enhancement. FDA Cleared 510K, OTC, Class II medical Device that is cleared for:
"The temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasms, minor strains and sprains; and muscle relaxation." No other claims are made or implied.
Arthritis Pain Get the help you need to understand what causes it and what you can do to ease it.
When joint cartilage wears away, bone rubs against bone, causing osteoarthritis. People with Rheumatoid Arthritis RA generally have inflammation that is generally what’s driving their pain. A rheumatologist can help manage their pain to get inflammation under control but if your RA inflammation is well managed and you are still having pain then there are other options available such as Advanced Heat Therapy and Vascular Circulation Enhancement. The Avacen 100 has been cleared for the temporary relief of pain associated with arthritis. *30 Day 100% money back guarantee.
The AVACEN 100 is the answer for many people who need or prefer an easy to use, drug-free alternative for temporary relief of pain, which might be associated with a variety of chronic and acute medical conditions
ATM (AVACEN TREATMENT METHOD)
Increasing your body’s blood temperature from within your vascular network, rather than from outside with the use of a spa, sauna, or heating pad is the key to the entire process. The palm of the hand is one of the best places for applied heat therapy because of the unique network of blood vessels that exists very close to the skin.
MICROCIRCULATION
Getting your exercise and increasing your heart rate on a regular basis are obviously very good for your body.
One of the reasons increased circulation is so good for you has to do with a lesser talked about subject of naturally increased microcirculation.
While circulation refers to blood flow to and from vital organs, microcirculation refers to blood flow in the smallest blood vessels in the body – capillaries, arterioles, and other such blood vessels. These blood vessels are often embedded in the organs, including the skin, and interact directly with muscle tissue.
Poor microcirculation is one of the single biggest contributing factors to almost all health problems: Diabetes, hypertension, vascular disease, atherosclerosis, kidney disease, Alzheimer’s, early aging and others. It is estimated that 80% of the population over the age of 40 may have moderately to extremely serious microcirculation problems and almost every non-injury related pain can be traced to a compromised microcirculation issue.
Until recently, there has never really been an increase in a body’s microcirculation without a proportionally larger increase in the body’s circulation. Meaning, you couldn’t really get the benefits of increased microcirculation without exercising, doing yoga, working out, going in a sauna or jacuzzi, stretching, water aerobics, or other activities that might get the blood flowing.
Microcirculation just may be exactly what your body needs to reduce pain on a daily basis.
3 US Patents. * International Patents. Additional patents Pending.
Get Complete details and Brochure
Additional Research and Clinical Trials have been conducted for other possible future applications.
Investigation of Thermal Exchange System for Fibromyalgia Pain
Validated through a study conducted by The Department of Veteran Affairs and the University of California, San Diego between 2001-2013. Results demonstrate that ATM significantly reduces the widespread pain associated with FMS - 20 times more effectively than FDA approved drugs. The Avacen is NOT CLEARED FOR FIBROMYALGIA AND IS ONLY cleared in the US only for "The temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasms, minor strains and sprains; and muscle relaxation."

SAN DIEGO, CA – October 11, 2017 - AVACEN Medical, Inc. (AVACEN)
announced that Frost & Sullivan has recognized the company with their 2017 New Product Innovation Award for its fibromyalgia pain management device. The award is based on extensive primary and secondary medical device market research by Frost & Sullivan’s industry experts.
AVACEN Medical, through its first-to-market OTC, drug-free pain relief product, the AVACEN 100, offers a safe and effective solution with a unique approach to treating fibromyalgia widespread pain. Brahadeesh Chandrasekaran, healthcare industry analyst with Frost & Sullivan and contributor to Forbes, the BBC, and most recently keynote speaker at the 2017 Boston BIOMEDEVICE conference on the "Future of the Medical Device Industry" recently stated: “The key AVACEN 100 benefits for patients are cost effectiveness, ease of use, and systemic treatment.” He continued, “Some of the major drawbacks with existing devices in the market are either they are not easy to use or are invasive in nature, especially for patients above 65 years of age. Some require constant supervision and are generally expensive. Further, existing non-invasive devices such as pulsed electromagnetic fields do not have the capacity to penetrate deeply enough throughout the entire human body and are not systemic.” “We are grateful to be recognized by Frost & Sullivan, whose analysts closely track our industry”, stated AVACEN Medical CEO, Thomas Muehlbauer. He added, “To be grouped with other 2017 award recipients such as Amazon, GE Healthcare, and Phillips Healthcare is even more of an honor.” Prior to the AVACEN 100, the only way to systemically treat the widespread pain associated with fibromyalgia was to use drugs such as Lyrica, Cymbalta, or Savella. The AVACEN 100 has been successfully helping those with widespread pain associated with fibromyalgia in the European Union. Fibromyalgia-diagnosed Amanda in Essex, England commented, “I had difficulty driving, getting dressed and washing my hair, experiencing no sleep at all, tremendous pain, with every movement sending my body in painful spasm…I cannot recommend [the AVACEN 100] enough, how this has and is helping me… the physio will be amazed with my progress.”
AVACEN Medical, 9835 Carroll Centre Road, Suite 104, San Diego, CA 92126 888-428-2236
Fibromyalgia has recently been thrust into the spotlight by Lady Gaga revealing she has been living with the disorder, which is characterized by chronic widespread pain. As per the National Fibromyalgia Association, fibromyalgia affects an estimated 10 million people in the United States and an estimated 3-6% of the global population. The incidence rises with age, so that by age 80, approximately 8% of adults meet the American College of Rheumatology classification of fibromyalgia. It has a devastating impact on those who suffer from the disease and their personal support systems. In addition, it imposes a large economic burden on public and private healthcare providers.
The AVACEN 100 is a Class II medical device that uses the AVACEN Treatment Method. It recently received CE (Conformité Européenne) mark and Health Canada approval to treat widespread pain associated with fibromyalgia. CE marking allows AVACEN to market the AVACEN 100 to the European Union’s 28 member countries where many prescription drugs available in the U.S. have been rejected by regulatory officials for treating fibromyalgia pain. The AVACEN Treatment Method is an entirely new concept in chronic pain treatment that noninvasively and safely infuses heat into the circulatory system to create muscular relaxation while increasing microcirculation throughout the body. Unlike other medical devices providing local pain relief, the AVACEN 100’s unique mechanism of action offers widespread pain relief. The AVACEN 22-person fibromyalgia study was conducted under a Cooperative Research Development Agreement with the U.S. Department of Veterans Affairs at the University of California San Diego Center for Pain Medicine, Perlman Medical Center, and the VA Medical Center, San Diego. The 28-day AVACEN follow-up study produced a statistically significant reduction of over 40% in the widespread pain index and a reduction in average tender point counts from approximately 15 to 9 (11 is used for clinical assessment). Study details can be found at https://www.avacen.com/fms and are also published on the National Institutes of Health – ClinicalTrials.gov website.
BREAKING NEWS SAN DIEGO, DECEMBER 23, 2016
PRNewswire/ -- AVACEN Medical (AVACEN) announced its AVACEN 100, Class-IIa, OTC medical device has received the CE (Conformité Européenne) Mark approval to treat widespread pain associated with fibromyalgia. The CE Mark allows AVACEN to market the AVACEN 100 to the European Union's 28 member countries where many prescription drugs, available in the U.S., have been rejected by regulatory officials for treating fibromyalgia pain.
CE approval for fibromyalgia treatment was based on a Clinical Evaluation Report, which highlighted AVACEN's very promising 22-person fibromyalgia study. One hundred percent (100%) positive results were recorded for the full therapeutic treatment group. The AVACEN study was conducted under a Cooperative Research Development Agreement with the U.S. Department of Veterans Affairs at the University of California San Diego Center for Pain Medicine, Perlman Medical Center and the VA Medical Center, San Diego.
Comparing the AVACEN clinical study active-group results to 2015 NPF survey:
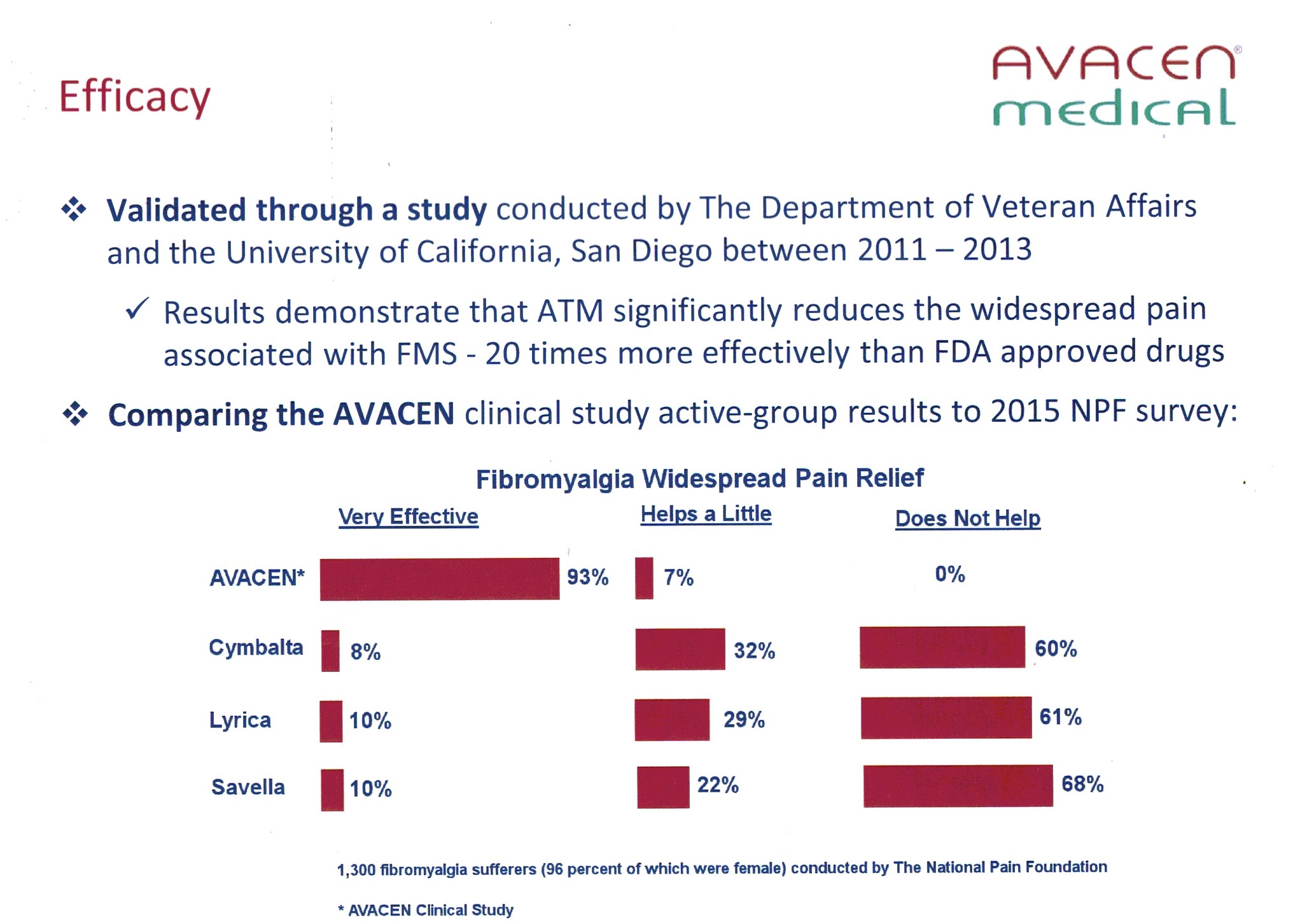
Avacen is cleared only for temporary arthritis and muscle pain relief, and muscle relaxation in the United States.
Fibromyalgianewstoday Studies
Several other clinical studies have produced scientifically significant results:
Conducted by The Department of Veterans Affairs & The University of California, San Diego RESULTS: A statistically significant reduction in widespread pain.
STUDY: Cardiovascular Effects of ATM Heat/Negative Pressure Conducted by San Diego State University RESULTS: A statistically significant reduction in “Mean Arterial Pressure”.
IMPORTANT NOTES: The AVACEN 100 is not for sale in the U.S. or E.U. for any non-cleared or non-approved indication mentioned in this document.
E.U. CE-Approval: The AVACEN 100 is a heat therapy system indicated for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasms, minor strains and sprains; the temporary relief of widespread pain associated with fibromyalgia; muscular relaxation; and the temporary increase of microcirculation.
U.S. FDA-Clearance: The AVACEN 100 is FDA-cleared as a heat therapy system indicated for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis; muscle spasms; minor strains and sprains; muscular relaxation; and the temporary increase of local circulation where applied.
Besides avoiding the detrimental effect of possible damage to the heart or liver by some pain medications you may want to try the AVACEN because of an insufficient response to pain medications, as a drug-free alternative if you are pregnant, nursing, or pre-pregnancy, or if you simply are looking for a healthy and homeopathic solution. You still need to consult with a Doctor before using the AVACEN 100 if you are under 18 years of age, are pregnant, have a history of heart disease, blood circulation problems, have a temperature higher than 99.5ºF or any other medical concerns.
The AVACEN 100 and The AVACEN Treatment Method (ATM) were invented and developed by AVACEN Medical of San Diego, California. Both the AVACEN 100 and AVACEN Treatment Method are protected by one or more of the following U.S. patents: 8,679,170; 9,066,781, 9,192,509 and international equivalents. Affiliate ID 253906
Videos
-

Dr Nathan Newman MD AVACEN 100
This video is about Dr Nathan Newman MD AVACEN 100
-

Dr Tony Allina Avacen 100
This video is about Dr Tony Allina Avacen 100
-

AVACEN 100- Chronic pain, Arthritis, Joint Treatment FDA Approved
Does this device really work? AVACEN Medical: Arthritis, Joint...
-

Avacen 100 Proving Results with a Capillary Microscope Pt 1
Does this medical device really work? *I am selling my Demo u...
-

BP 144 over 92 after the Avacen
Blood pressure test after the Avacen. BP down 36 points after ...
-

BP 180 over 92 before using the Avacen
Blood Pressure tests before the Avacen
-

Avacen 20 minute session for blood pressure test
-

Cappilary Blood Flow after the avacen
Cappilary Blood Flow after a 20 minute avacen session




























