
Hydrogen Oxygen Nanobubble Bath Clinical Study
2017; 7: 9268.
Published online 2017 Aug 24. doi: 10.1038/s41598-017-08988-7
PMCID: PMC5570893
PMID: 28839175
Oxygen nanobubbles revert hypoxia by methylation
programming
Pushpak N. Bhandari,1,4 Yi Cui,1,4 Bennett D. Elzey,2 Craig J. Goergen,3 Christopher M.
Long,1,4 and Joseph Irudayaraj 1,4
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Associated Data
Supplementary Materials
Go to:
Abstract
Targeting the hypoxic tumor microenvironment has a broad impact in
cancer epigenetics and therapeutics. Oxygen encapsulated nanosize
carboxymethyl cellulosic nanobubbles were developed for mitigating the
hypoxic regions of tumors to weaken the hypoxia-driven pathways and
inhibit tumor growth. We show that 5-methylcytosine (5mC)
hypomethylation in hypoxic regions of a tumor can be reverted to
enhance cancer treatment by epigenetic regulation, using oxygen
nanobubbles in the sub-100 nm size range, both, in vitro and in vivo.
Oxygen nanobubbles were effective in significantly delaying tumor
progression and improving survival rates in mice models. Further,
significant hypermethylation was observed in promoter DNA region of
BRCA1 due to oxygen nanobubble (ONB) treatment. The nanobubbles
can also reprogram several hypoxia associated and tumor suppressor
genes such as MAT2A and PDK-1, in addition to serving as an ultrasound
contrast agent. Our approach to develop nanosized oxygen encapsulated
bubbles as an ultrasound contrast agent for methylation reversal is
expected to have a significant impact in epigenetic programming and to
serve as an adjuvant to cancer treatment.
Go to:
Introduction
Epigenetics plays an important role in regulating the expression of genes
and corresponding cellular and molecular pathways1. DNA methylation
(i.e. covalent addition of a methyl group to the C-5 carbon of the cytosine
group in DNA) constitutes an important step in epigenetic programming
and has been implicated in gene expression2–4. Addition of methyl groups
to the cytosine derivatives in the DNA sequence can render the
associated genes transcriptionally inactive5. DNA demethylation can lead
to a hypomethylated state, but is counteracted by active DNA
methylation to achieve a balanced methylation level6, 7. In neoplasia, the
unregulated proliferation of cellular mass, without a sustainable rate of
angiogenesis, leads to the development of hypoxic conditions inside the
tumor8. In response to the pervasive hypoxic environment, several
oncogenic processes occur in the cells, one of which is epigenetic
alterations, resulting in an increase in tumor growth and survival of
cancer cells9, 10. These alterations include global hypomethylation
(primarily of oncogenes rendering them active)11, gene-specific
hypermethylation (of CpG islands in the promoter regions of tumor
suppressor genes, rendering them inactive), and inducing cell
proliferation via dysregulated cell growth3. Ten-eleven translocation
(TET) enzymes are a group of Fe2+ and α-ketoglutarate dependent
dioxygenases that oxidize the conversion of 5mC to 5hmC and other
downstream derivatives12–14. In mammalian cells, TET enzyme is the only
characterized factor mediating the active DNA demethylation process12, 13.
The activity of TET enzymes that have been shown to catalyze DNA
demethylation is also limited by oxygen supply14. Although epigenetic
therapy in the laboratory and clinics have largely focused on changes at
gene promoters15, 16, epigenetic abnormalities such as DNA 5mC
methylation across the genome17 are now being looked upon as
diagnostic (tumor staging, outcome prediction, and malignancy) and
therapeutic targets (epigenetic drugs). Regulation of the hypoxic
microenvironment and epigenetic events are promising steps in
anticancer therapies because several hypoxia18–20 and epigenetic15, 21–
23 targeted therapies have shown efficacy in the clinic24–26. Further, this
view is also supported by findings on the effect of supplemental oxygen
that weaken the hypoxia-driven pathways to improve cancer
immunotherapy to promote tumor regression27.
Supplemental respiratory oxygen has shown significant lung tumor
regression and long-term survival in mice and is being proposed as a
treatment option27–29 by combining it with existing cancer
immunotherapies. However, the toxicity and nonspecific inflammatory
response30, 31 in addition to its large instrumentation footprint, diminishes
its potential as a viable therapeutic option. Hence the motivation to
develop injectable and safer treatment options that weaken the hypoxiadriven
global hypomethylation and hypoxia-adaptive pathways. Herein,
we reason that delivery of nanosize oxygen bubbles specifically to
hypoxic regions would help to regulate the epigenetic state by
destabilizing the hypoxia-mediated pathways such as hypoxia inducible
factor (HIF)32 that promote tumor progression. Global 5mC methylation
along with HIF-1α levels were monitored in vitro and in vivo during the
course of tumor regression upon hypoxia reversal, using human cervical
cancer (HeLa) and murine bladder cancer (MB49) tumor models.
Further, promoter methylation analysis was used to assess a group of
tumor suppressor genes. Our results indicate that the oxygen
nanobubbles can potently alter the epigenetic state of the cell cyclerelated
genes and mitigate cancer cell proliferation. Our approach
provides an injectable, nano-scale oxygen delivery platform to mitigate
hypoxia and to alter the epigenetic state, thus providing an opportunity
for epigenetic therapy approaches by destabilizing the hypoxia-adaptive
pathways in the tumor.
Go to:
Results
We hypothesize that alteration of DNA hypomethylation in hypoxic
cancer cells can be achieved by the delivery of oxygen to the cellular
microenvironment with nanosize oxygen bubbles (Fig. 1a,b). In
particular, our approach consists of encapsulating oxygen inside a
sodium carboxymethylcellulose polymeric shell (Fig. 1a) to form
nanobubbles 100–200 nm in diameter (Fig. 2a,b) by a crosslinking step33.
High resolution TEM micrographs show that the synthesized
nanobubbles have a spherical shape (Fig. 2a) and contain an oxygen core
at the center and a ~50 nm carboxymethyl cellulose shell encapsulating
the nanobubble. Dynamic light scattering (DLS) shows that the size
distribution of nanobubbles is in the range between 50–200 nm with a
normal distribution centered around 70 nm (Fig. 2b). Further, sodium
carboxymethylcellulose is a commercially used, FDA-approved
pharmaceutical excipient. Upon uptake, the acidic microenvironment
around and inside the tumor cells34 will cause the nanobubble shells to
disintegrate, thereby increasing the cellular oxygen levels. We expect the
release of oxygen inside the hypoxic cells will destabilize the hypoxiaadaptive
pathways and reprogram the cellular epigenome to attain
normal DNA methylation levels, or cause global hypermethylation. The
targeted oxygen delivery is also expected to promote the regression of
tumor growth in the hypoxic xenografted MB49 (bladder cancer) and
HeLa (cervical cancer) tumors.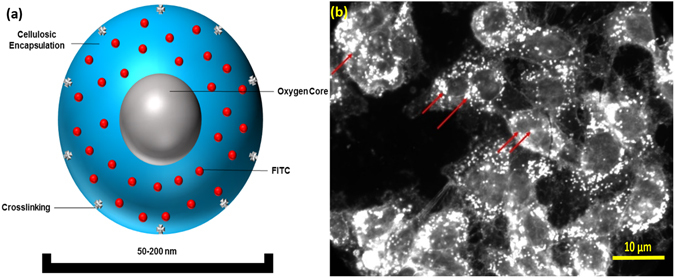
Figure 1
Oxygen Nanobubble configuration and mechanism of 5mC hypermethylation. (a) Schematic
representation of oxygen nanobubble; (b) Oxygen nanobubbles (red arrows) localize
within HeLa cells in the cytoplasm as well as the nucleus. Significantly enhanced dark field
microscopy images are provided. Scale bar = 10 μm
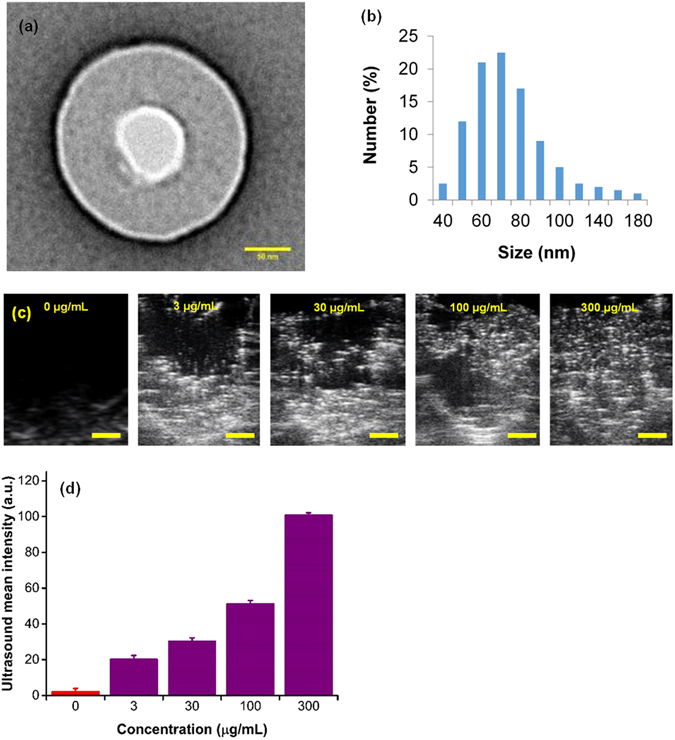
In vitro characterization, hypoxia reprogramming, and imaging of nanobubbles. (a) TEM image of nanobubble with an oxygen compartment at the core surrounded by sodium carboxymethylcellulose shell. Scale bar = 50 nm. (b) Dynamic light scattering (DLS) size distribution of nanobubbles. (c) Ultrasound images of the corresponding signal generated from varying concentrations of nanobubbles (0–300 µg/mL). The contrast generated is due to oxygen trapped inside nanobubbles. Scale bar = 1 mm. (d) Graph displaying averaged mean grey scale intensity corresponding to increasing concentrations of nanobubbles (0–300 µg/mL). The results are mean values from three independent experiments. Error bars represent ± s.d. Note that there is a significant linear relationship between mean ultrasound gray scale intensity and concentration of nanobubbles (see Supplementary Fig. 1).
In addition to reoxygenation, we anticipate that oxygen nanobubbles will
act as contrast agents for ultrasound imaging. Nanobubbles also possess
unique light scattering and absorption characteristics as demonstrated
using dark field microscopy (Fig. 1b) and in our prior work33. To test the
ultrasound imaging intensity response to increasing concentration of
nanobubbles, agarose gel molds were prepared with varying
concentration of nanobubbles (Supplementary Fig. 1). B-mode ultrasound
images of injected nanobubbles are shown in Fig. 2c and the
corresponding mean gray scale intensity measurements are depicted in
Fig. 2d. A linear increase in ultrasound grey scale imaging intensity
(Fig. 2d and Supplementary Fig. 2) was observed as a function of
nanobubble concentration (R2 = 0.95) in the concentration range
evaluated (0–300 μg/mL). Further, HeLa cell cultures grown in tissue
culture plates were incubated either with oxygen nanobubbles or
phosphate buffer saline (PBS). After incubation for 24 h, the cell cultures
were imaged using a 256-element 22–55 MHz ultrasound transducer
with a center frequency of 40 MHz (for sample preparation and imaging
details, see Methods Section). Images show that the spherical
nanobubbles are suspended in the media as well as around the HeLa
cells adhered to the bottom of the plate (Supplementary Fig. 3b)
compared to the HeLa cell culture without nanobubbles (Supplementary
Fig. 3a). The ultrasound gray scale imaging intensity in cell cultures with
nanobubbles was significantly higher than the control without the
addition of nanobubbles (Supplementary Fig. 4). The proposed design
allows for customization of its size to accommodate various oxygen
carrying capacity capable of generating different ultrasound contrast
intensity.
The 5mC levels in the hypoxic regions have been shown to rapidly
decrease, independent of the cell proliferation cycle35. In our
experiments, DNA was extracted after 48 h of incubation following a
factorial experiment design (data not shown) to ensure sufficient time
for the methylation changes to take effect35. Colorimetric enzyme-linked
immunosorbent assay (ELISA) was used to quantify 5mC levels36 after the
exposure of nanobubbles to a hypoxic environment (Fig. 3a,c) and the
5mC levels were further validated using liquid chromatography-mass
spectrometry (LC-MS/MS) (Fig. 3b,d). The DNA methylation levels
measured from cells exposed to ONBs for different time periods
(Fig. 3a,b) showed a distinct decrease (α = 0.05) in the DNA methylation
levels in hypoxic cells compared to the control. Further, irrespective of
the time of dose (start of treatment at 0 h or 24 h), no significant
difference was observed in the methylation levels (Fig. 3a,b and
Supplementary Fig. 6). However, in cells treated with nanobubbles, i.e.
addition of nanobubbles (0.5 mg/mL) at 0 h and after 24 h of incubation,
a rapid and significant increase in 5mC DNA methylation levels was
observed. Under hypoxia, DNA 5mC levels (measured as OD450
absorbance) linearly increased (P < 0.004, R2 = 0.58) corresponding to an
increase in oxygen nanobubble concentration (Fig. 3c,d and
Supplementary Fig. 5). A significant difference was observed (Fig. 3c,d)
between 0, 0.1, and 1 mg/mL of nanobubble concentration (α = 0.05).
The trends for different treatment conditions and oxygen nanobubble
concentrations were similar and validated by ELISA and LC-MS/MS. Our
observations infer that active 5mC levels in hypoxic tumor cells can be
increased using oxygen nanobubbles in a dose-dependent manner, in
vitro.
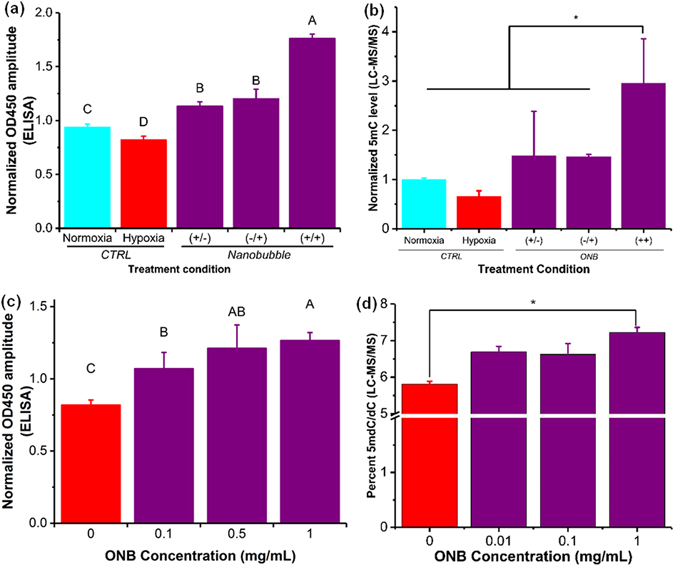
ONBs perturb 5mC hypomethylation in vitro. (a) 5mC methylation levels in HeLa cells as measured using ELISA for varying treatments, and 0.5 mg/mL nanobubble concentration, to identify the relation between treatment frequency and the total time of incubation. (+/−) Signifies samples with addition of nanobubbles 0 hours after incubation and no addition of nanobubbles after 24 hours of incubation. (−/+) Signifies the samples with no addition of nanobubbles after 0 hours of incubation but addition of nanobubbles after 24 hours of incubation. (+/+) Signifies samples with the addition of nanobubbles after both 0 hours and 24 hours of incubation. (b) 5mC methylation levels as measured using ELISA for varying concentration of nanobubble treatments under hypoxia for HeLa cells. The nanobubble treatment volume and time of treatment was the same for all samples. (c) Normalized 5mC methylation levels in HeLa cells as measured using LC-MS/MS for varying treatments, and 0.5 mg/mL nanobubble concentration, to identify the relation between treatment frequency and the total time of incubation. (+/−) Signifies samples with addition of nanobubbles 0 hours after incubation and no addition of nanobubbles after 24 hours of incubation. (−/+) Signifies the samples with no addition of nanobubbles after 0 hours of incubation but addition of nanobubbles after 24 hours of incubation. (+/+) Signifies samples with the addition of nanobubbles after both 0 hours and 24 hours of incubation. Samples were analyzed using LC-MS/MS. (d) 5mC methylation levels in HeLa cells as measured using LC-MS/MS for varying concentration of nanobubble treatments under hypoxia for HeLa cells. The nanobubble treatment volume and time of treatment was the same for all samples. The results are mean values from three independent experiments ± s.d. Mean values not connected by same letter are significantly different. Significance established with one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. *P < 0.05.
There are no products listed under this category.


