Summer Body Red Light Pads
2 Summer Body Red Light Pads - Medium Plus Facial Pad
- SKU:
- summerbodyredlightpadmediumwithfacial
- Weight:
- 5.00 LBS
Description
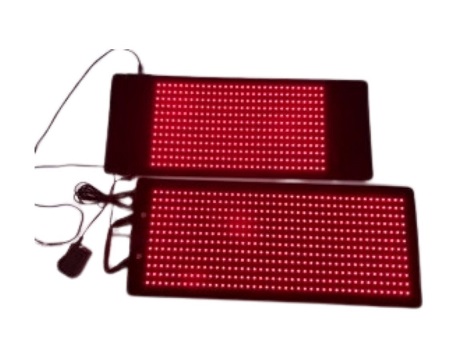
Summer Body Red Light Pad + Facial Pad with Near Infrared
2 Pads for the Belly and Face simultaneously in just 20 minutes
"New German Diodes" Medium Pad 11 x 29 + Facial Mask 11 x 29
1008 Diodes 360 Diodes
Introducing the new Red Light Body Pad System to rejuvenate your belly and face in just 20 minutes. Summer Body incorporates advanced LED technology in the Summer Body Pads with new more effective diodes from Germany that are the most powerful on the market. Using the power of Light Therapy, our system is the natural and healthy way to wrap your waist, hips, thighs, arms, and chin in Red Light and NIR Infrared for the fastest results possible and at an affordable price.



Whole body light therapy has no negative side effects, is completely safe. The treatment itself should be done every other day until the results you desire are achieved. The length of time is determined on whether the therapy itself.
12- - 24 sessions recommended.
Package includes: Summer Body Medium Pad with Power Supply
Pad Size 11 x 29
1008 Red Lights, 504 Near-Infrared Lights 1512 x 2 = 3024 Total Diodes)
Strongest Body Pad Diodes Available
Facial Pad 360 Red Lights, 720 Near Infrared Lights (1080 Diodes)
3 Year Warranty
Build to ISO 60601 requirements
ISO 13485 compliant ISO International Medical manufacturing supplier
890.55 Regulation Number
zero EMF
-
SAFETY
Red Light Therapy or Low Level Laser Therapy is considered a general wellness product under FDA 21 CFR and is intended only for general wellness use and presents a very low risk to users’ safety. This system encourages a general state of health or a healthy activity and is part of a exercise and diet program and promotes a healthy lifestyle. FDA 510K premarket notification is not required.
§890.5500 Infrared lamp. (a) Identification. An infrared lamp is a device intended for medical purposes that emits energy at infrared frequencies (approximately 700 nanometers to 50,000 nanometers) to provide topical heating. (b) Classification. Class II (performance standards).
(b) Classification. Class I (general controls). The device is exempt from the premarket notification procedures in subpart E of part 807 of this chapter, subject to the limitations in §890.9.
FDA Intended Use
• Restoration of Motion to Joints • Redevelop Muscles • Adjunct to Obesity as part of a diet and exercise program• Relaxation of muscles and relief from muscle spasms • Temporary relief of minor muscle and joint aches, pain and stiffness • Temporary relief of minor pain and stiffness associated with Arthritis • Temporarily increase blood circulation


















